nf-core/cageseq
CAGE-sequencing analysis pipeline with trimming, alignment and counting of CAGE tags.
22.10.6.
Learn more.
Introduction
This document describes the output produced by the pipeline. Most of the plots are taken from the MultiQC report, which summarises results at the end of the pipeline.
The directories listed below will be created in the results directory after the pipeline has finished. All paths are relative to the top-level results directory.
Pipeline overview
The pipeline is built using Nextflow and processes data using the following steps:
1. Raw read QC
FastQC gives general quality metrics about your sequenced reads. It provides information about the quality score distribution across your reads, per base sequence content (%A/T/G/C), adapter contamination and overrepresented sequences.
For further reading and documentation see the FastQC help pages.
This step can be skipped via --skip_initial_fastqc.
NB: The FastQC plots displayed in the MultiQC report shows untrimmed as reads. They may contain adapter sequence and potentially regions with low quality. In parallel it also shows the FastQC results for the trimmed reads (marked with the suffix
.g) To see how your reads look after trimming, look at the FastQC reports in thetrimmed/directory.
Output directory : results/fastqc
*_fastqc.html: FastQC report containing quality metrics for your untrimmed raw fastq files.zips/*_fastqc.zip: Zip archive containing the FastQC report, tab-delimited data file and plot images.
2. Trimming
Cutadapt finds and removes adapter sequences, primers, poly-A tails and other types of unwanted sequence from your high-throughput sequencing reads.
By default this pipeline trims the cut enzyme binding site at the 5’-end and
linkers at the 3’-end (can be disabled by setting --trim_ecop or --trim_linkers to false).
Furthermore, to combat the leading-G-bias of CAGE-seq, G’s at the 5’-end are removed. Additional artifacts generated in the sequencing process, can be removed via the --trim_artifacts parameter.
All the following trimming process are skipped if --skip_trimming is supplied and the fastq files below are only available if ‘—save_trimmed’ is supplied.
Output directory: results/trimmed
adapter_trimmed/sample.adapter_trimmed.fastq.gz- FastQ file after removal of linkers and EcoP15 site.
adapter_trimmed/logs/- Trimming report (describes which parameters were used)
- if
--trim_5g:g_trimmed/sample.g_trimmed.fastq.gz- 5’ G-corrected FastQ file
g_trimmed/logs/- Trimming report (describes which parameters were used)
- if
--trim_artifacts:artifacts_trimmed/sample.artifact_trimmed.fastq.gz- FastQ file after artifact removal
artifacts_trimmed/logs/- Trimming report (describes which parameters were used)
3. Ribomosal RNA removal
SortMeRNA is a program for filtering, mapping and OTU-picking NGS reads in metatranscriptomic and metagenomic data.
The MultiQC report shows the overall percentage of rRNA in the sample in the general statistics section. The SortMeRNA section shows a bar plot of the filtered rRNA types.
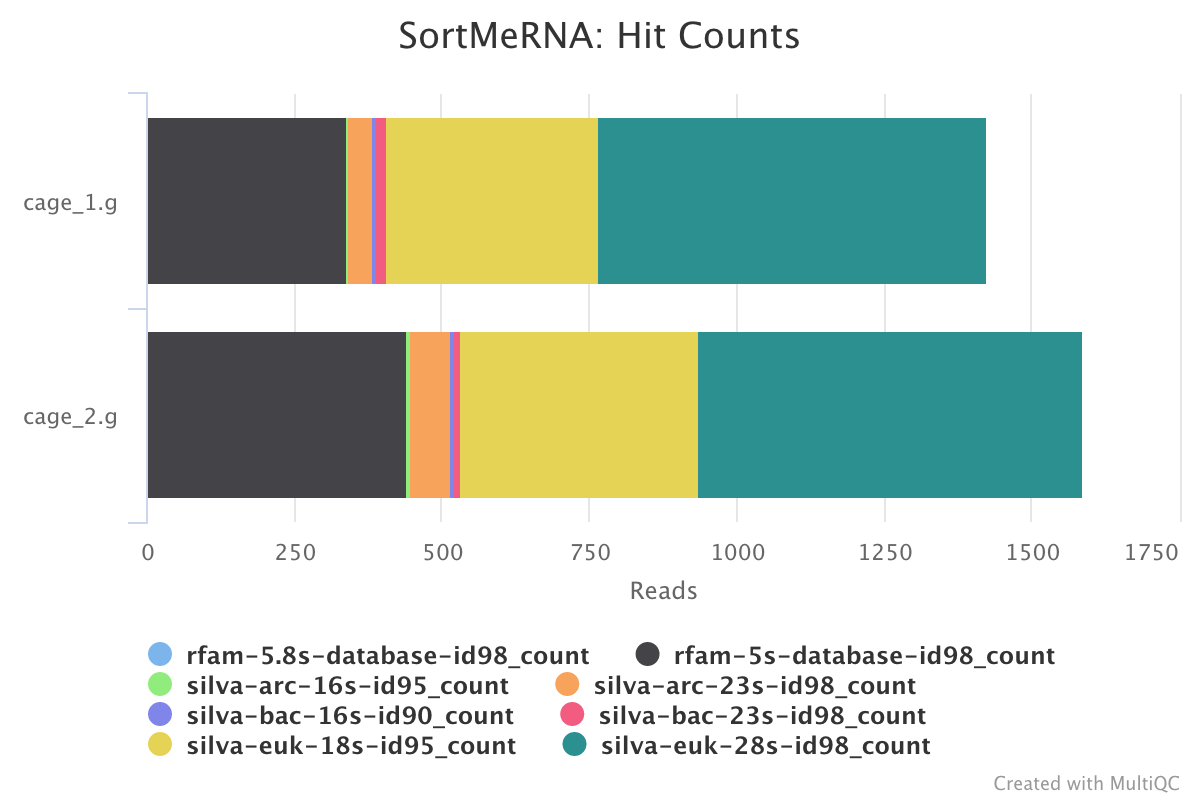
Output directory : results/SortMeRNA/
logs/- ribosomal RNA mapping reports (describes which parameters were used)
4. Alignment
The reads are aligned either with STAR or with bowtie, set via --aligner.
STAR
STAR is a read aligner designed for RNA sequencing. STAR stands for Spliced Transcripts Alignment to a Reference.
The STAR section of the MultiQC report shows a stacked bar plot with alignment rates: good samples should have most reads as Uniquely mapped and few Unmapped reads.
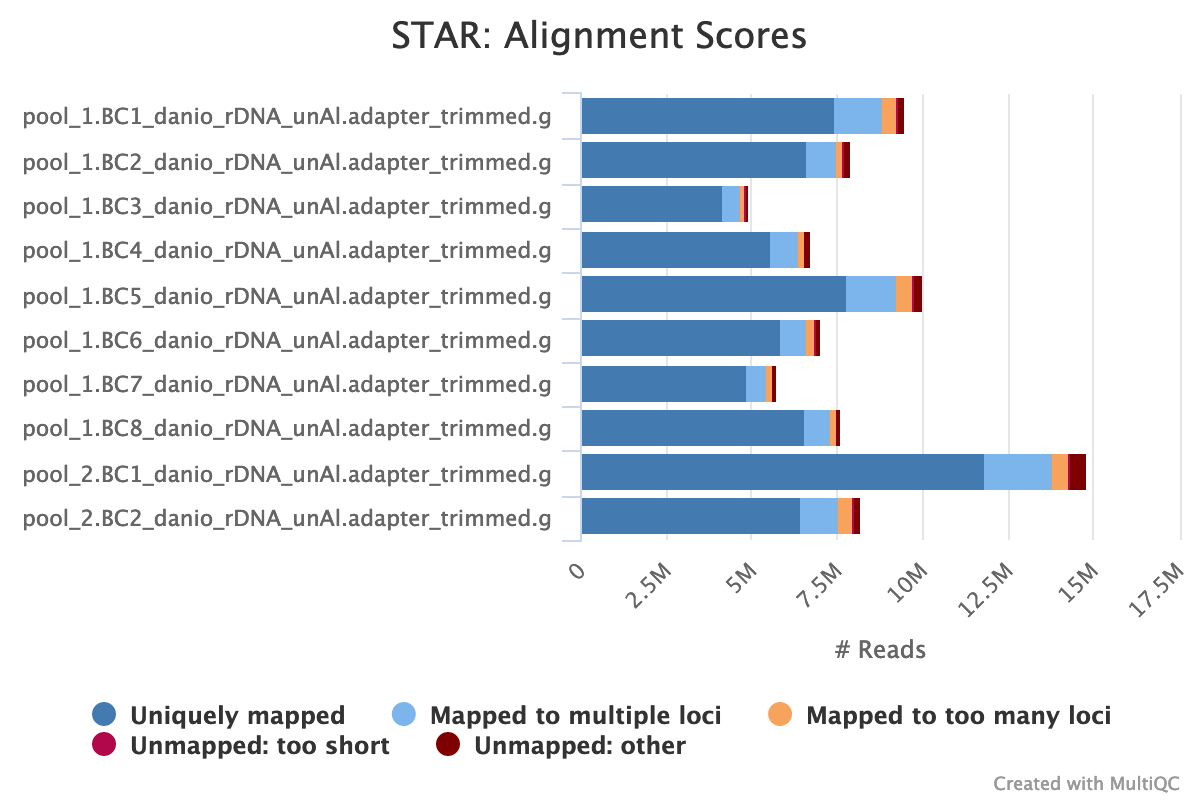
Output directory: results/STAR
Sample_Aligned.sortedByCoord.out.bam- The aligned BAM file
Sample_Log.final.out- The STAR alignment report, contains mapping results summary
Sample_Log.outandSample_Log.progress.out- STAR log files, containing a lot of detailed information about the run. Typically only useful for debugging purposes.
Sample_SJ.out.tab- Filtered splice junctions detected in the mapping
Bowtie 1
Bowtie 1 is an ultrafast, memory-efficient short read aligner.
The bowtie 1 section of the MultiQC report shows a stacked bar plot with alignment rates: good samples should have most reads as aligned and few Not aligned reads.
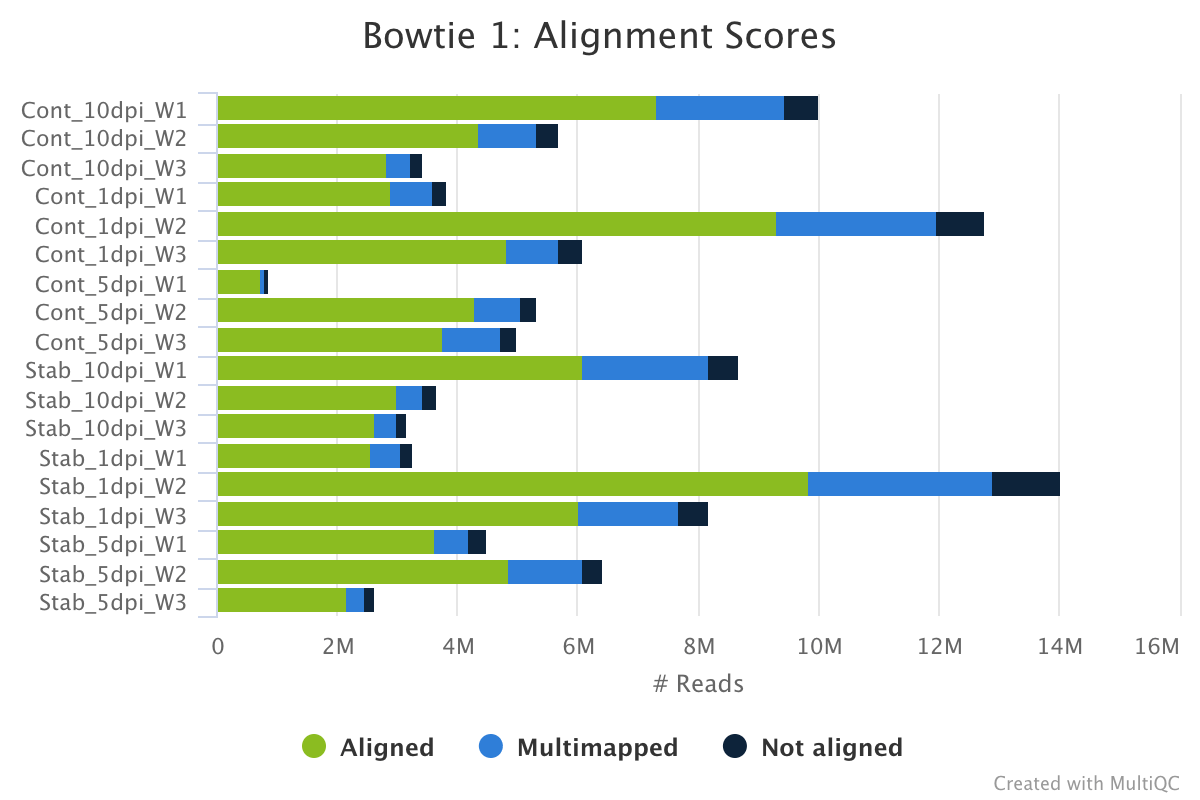
Output directory: results/bowtie
Sample.bam- The aligned BAM file
logs/Sample.out- The bowtie alignment report, contains mapping results summary
5. CAGE tag grouping
The custom script bin/make_ctss.sh generates a bed file (and a bigWig file with --bigwig) for each sample with the summed up 1bp unclustered cage tags.
Output directory: results/ctss
Sample.ctss.bed- A BED6 file with the mapped cage tags
- if
--bigwig:Sample.ctss.bw- A bigWig file with the mapped cage tags
6. CTSS clustering
paraclu
paraclu finds clusters in data attached to sequences. It is applied on the pool of all ctss bed files to cluster and returns a bed file with the clustered cage-defined transcription start sites (CTSS).
Output directory: results/ctss/clusters
-
ctss_all_clustered_simplified.bedA BED6 file with the found clusters and their pooled expression as the score. -
ctss_all_clustered_simplified.bed- A BED6 file with the clustered CTSSs and their pooled counts
7. Count table generation
The cage tags are intersected with the clusters identified by paraclu and summarized in a count table.
Output directory: results/ctss/
count_table.tsv:- Each column of the count table stands for one sample and each row for one tag cluster. The first row of this table is the header with sample names and the first column contains the tag cluster coordinates.
8. QC of results
RSeQC
RSeQC is a package of scripts designed to evaluate the quality of RNA seq data. You can find out more about the package at the RSeQC website.
This pipeline only runs the read destribution RSeQC scripts on the CTSS clusters. The results are summarised within the MultiQC report.
read_distribution.py calculates how mapped reads are distributed over genomic features.
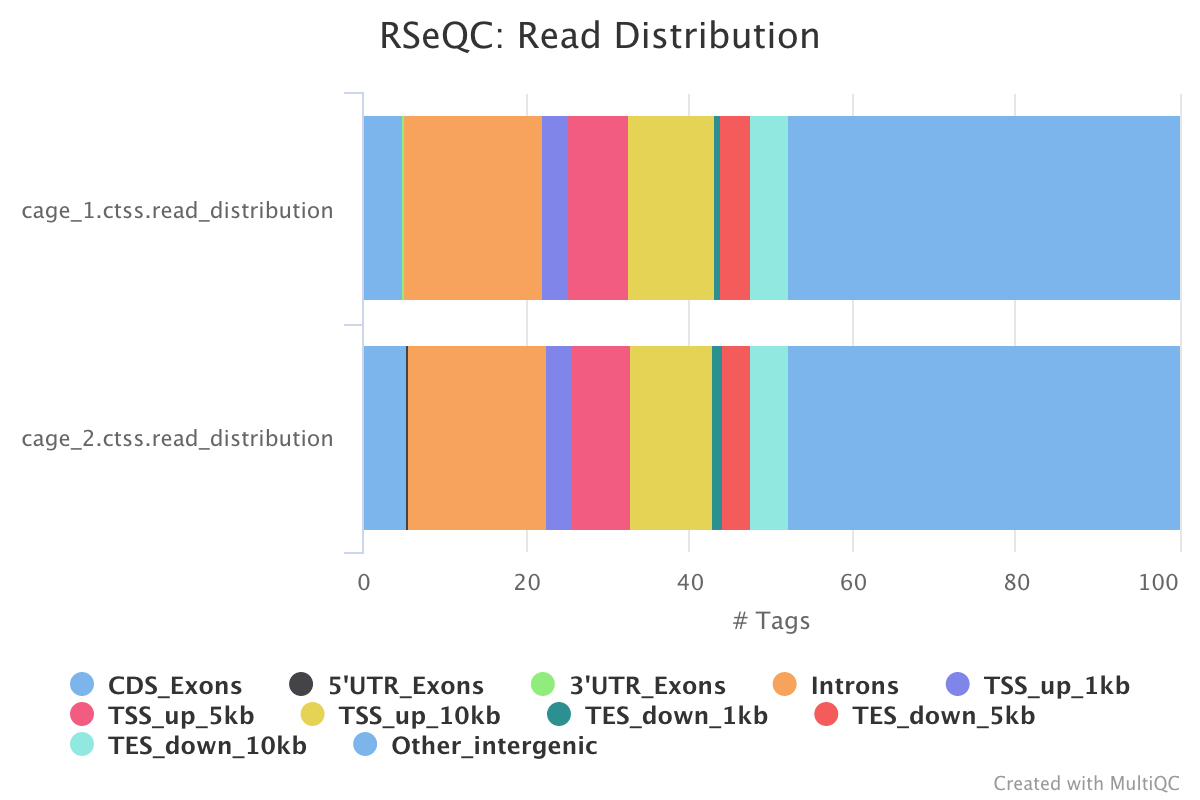
Output directory : results/rseqc/read_distribution/
- Sample_read_distribution.txt:
- text file with the raw data describing the distribution of the mapped reads.
MultiQC
MultiQC is a visualization tool that generates a single HTML report summarizing all samples in your project. Most of the pipeline QC results are visualised in the report and further statistics are available in the report data directory.
The pipeline has special steps which also allow the software versions to be reported in the MultiQC output for future traceability.
For more information about how to use MultiQC reports, see https://multiqc.info.
Output files:
multiqc/multiqc_report.html: a standalone HTML file that can be viewed in your web browser.multiqc_data/: directory containing parsed statistics from the different tools used in the pipeline.multiqc_plots/: directory containing static images from the report in various formats.
Pipeline information
Nextflow provides excellent functionality for generating various reports relevant to the running and execution of the pipeline. This will allow you to troubleshoot errors with the running of the pipeline, and also provide you with other information such as launch commands, run times and resource usage.
pipeline_info/- Reports generated by Nextflow:
execution_report.html,execution_timeline.html,execution_trace.txtandpipeline_dag.dot/pipeline_dag.svg. - Reports generated by the pipeline:
pipeline_report.html,pipeline_report.txtandsoftware_versions.csv. - Documentation for interpretation of results in HTML format:
results_description.html.
- Reports generated by Nextflow: