nf-core/mhcquant
Identify and quantify MHC eluted peptides from mass spectrometry raw data
2.4.1). The latest
stable release is
3.0.0
.
Introduction
nfcore/mhcquant is a bioinformatics analysis pipeline used for quantitative processing of data dependent (DDA) peptidomics data.
It was specifically designed to analyse immunopeptidomics data, which deals with the analysis of affinity purified, unspecifically cleaved peptides that have recently been discussed intensively in the context of cancer vaccines.
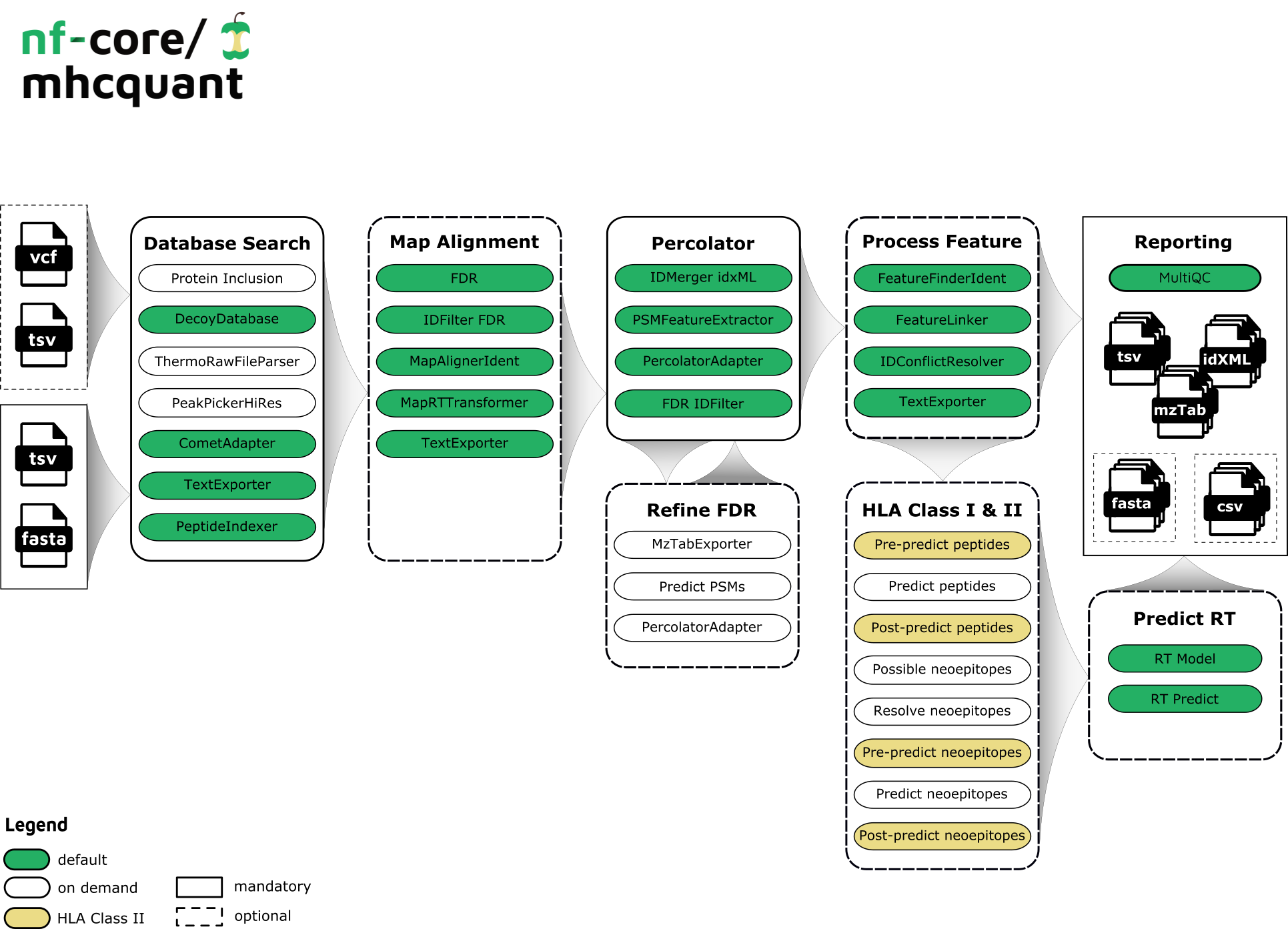
The workflow is based on the OpenMS C++ framework for computational mass spectrometry. RAW files (mzML) serve as inputs and a database search (Comet) is performed based on a given input protein database. FDR rescoring is applied using Percolator based on a competitive target-decoy approach (reversed decoys). For label free quantification all input files undergo identification based retention time alignment (MapAlignerIdentification), and targeted feature extraction matching ids between runs (FeatureFinderIdentification). In addition, a variant calling file (vcf) can be specified to translate variants into proteins that will be included in the database search and binding predictions on specified alleles (alleles.tsv) using MHCFlurry (Class 1) or MHCNugget (Class 2) can be directly run on the output peptide lists. Moreover, if a vcf file was specified, neoepitopes will automatically be determined and binding predictions can also directly be predicted for them.
The pipeline is built using Nextflow, a workflow tool to run tasks across multiple compute infrastructures in a very portable manner. It uses Docker/Singularity containers making installation trivial and results highly reproducible. The Nextflow DSL2 implementation of this pipeline uses one container per process which makes it much easier to maintain and update software dependencies. Where possible, these processes have been submitted to and installed from nf-core/modules in order to make them available to all nf-core pipelines, and to everyone within the Nextflow community!
On release, automated continuous integration tests run the pipeline on a full-sized dataset on the AWS cloud infrastructure. This ensures that the pipeline runs on AWS, has sensible resource allocation defaults set to run on real-world datasets, and permits the persistent storage of results to benchmark between pipeline releases and other analysis sources. The results obtained from the full-sized test can be viewed on the nf-core website.
Quick Start
-
Install
Nextflow(>=22.10.1) -
Install any of
Docker,Singularity(you can follow this tutorial),Podman,ShifterorCharliecloudfor full pipeline reproducibility (you can useCondaboth to install Nextflow itself and also to manage software within pipelines. Please only use it within pipelines as a last resort; see docs). -
Download the pipeline and test it on a minimal dataset with a single command:
nextflow run nf-core/mhcquant -profile test,YOURPROFILE --outdir <OUTDIR>Note that some form of configuration will be needed so that Nextflow knows how to fetch the required software. This is usually done in the form of a config profile (
YOURPROFILEin the example command above). You can chain multiple config profiles in a comma-separated string.- The pipeline comes with config profiles called
docker,singularity,podman,shifter,charliecloudandcondawhich instruct the pipeline to use the named tool for software management. For example,-profile test,docker. - Please check nf-core/configs to see if a custom config file to run nf-core pipelines already exists for your Institute. If so, you can simply use
-profile <institute>in your command. This will enable eitherdockerorsingularityand set the appropriate execution settings for your local compute environment. - If you are using
singularity, please use thenf-core downloadcommand to download images first, before running the pipeline. Setting theNXF_SINGULARITY_CACHEDIRorsingularity.cacheDirNextflow options enables you to store and re-use the images from a central location for future pipeline runs. - If you are using
conda, it is highly recommended to use theNXF_CONDA_CACHEDIRorconda.cacheDirsettings to store the environments in a central location for future pipeline runs.
- The pipeline comes with config profiles called
-
Start running your own analysis!
nextflow run nf-core/mhcquant -profile test,<docker/singularity/podman/shifter/charliecloud/conda/institute> \ --input 'samples.tsv' \ --fasta 'SWISSPROT_2020.fasta' \ --allele_sheet 'alleles.tsv' \ --predict_class_1 \ --refine_fdr_on_predicted_subset \ --outdir ./results
Pipeline summary
Default Steps
By default the pipeline currently performs the following
- Identification of peptides in the MS/MS spectra using comet (
CometAdapter) - Refreshes the protein references for all peptide hits and adds target/decoy information (
PeptideIndexer) - Estimates the false discovery rate on peptide and protein level (
FalseDiscoveryRate) - Filters peptide/protein identification results on ID based alignment (
IDFilter) - Converts XML format to text files (
TextExporter) - Merges several idXML files into one idXML file (
IDMerger) - Extract PSM features for Percolator (
PSMFeatureExtractor) - Facilitates the input to, the call of and output integration of Percolator (
PercolatorAdapter) - Filters peptide/protein identification result (
IDFilter)
Additional Steps
Additional functionality contained by the pipeline currently includes:
Input
- Inclusion of proteins in the reference database (
mhcnuggets,mhcflurry,fred2) - Create a decoy peptide database from standard FASTA databases (
DecoyDatabase) - Conversion of raw to mzML files (
ThermoRawFileParser) - Executing the peak picking with high_res algorithm (
PeakPickerHiRes)
Map alignment
- Corrects retention time distortions between maps, using information from peptides identified in different maps (
MapAlignerIdentification) - Applies retention time transformations to maps (
MapRTTransformer)
Refine FDR
- This application converts several OpenMS XML formats to mzTab. (
MzTabExporter) - Predict psm results using mhcflurry to shrink search space (
mhcflurry) - Facilitates the input to, the call of and output integration of Percolator (
PercolatorAdapter)
Process features
- Detects features in MS1 data based on peptide identifications (
FeatureFinderIdentification) - Group corresponding features across labelfree experiments (
FeatureLinkerUnlabeledKD) - Resolves ambiguous annotations of features with peptide identifications (
IDConflictResolver) - Converts XML format to text files (
TextExporter) - Annotates final list of peptides with their respective ions and charges (
IonAnnotator)
Prediction of HLA class 1 peptides
- Predict peptides (
mhcnuggets,mhcflurry,fred2) - Predict possible neoepitopes - when an vcf files is provided (
mhcnuggets,mhcflurry,fred2) - Predict neoepitopes based on the peptide hits (
mhcnuggets,mhcflurry,fred2) - Resolve found neoepitopes (
mhcnuggets,mhcflurry,fred2)
Prediction retention time
- Used to train a model for peptide retention time prediction or peptide separation prediction (
RTModel) - Retention Times Predictor Found Peptides and neoepitopes (
RTPredict)
Documentation
The nf-core/mhcquant pipeline comes with documentation about the pipeline usage, parameters and output.
- Nextflow installation
- Pipeline configuration
- Running the pipeline
- This includes tutorials, FAQs, and troubleshooting instructions
- Output and how to interpret the results
Credits
nf-core/mhcquant was originally written by Leon Bichmann from the Kohlbacher Lab. The pipeline was re-written in Nextflow DSL2 and is primarily maintained by Marissa Dubbelaar from Clinical Collaboration Unit Translational Immunology and Quantitative Biology Center in Tübingen.
Helpful contributors:
- Lukas Heumos
- Alexander Peltzer
- Maxime Garcia
- Gisela Gabernet
- Susanne Jodoin
- Oskar Wacker
- Leon Kuchenbecker
- Phil Ewels
- Christian Fufezan
- Sven Fillinger
- Kevin Menden
- Jonas Scheid
Contributions and Support
If you would like to contribute to this pipeline, please see the contributing guidelines.
For further information or help, don’t hesitate to get in touch on the Slack #mhcquant channel (you can join with this invite).
Citations
If you use nf-core/mhcquant for your analysis, please cite it using the following doi: 10.5281/zenodo.1569909 and the corresponding manuscript:
MHCquant: Automated and Reproducible Data Analysis for Immunopeptidomics
Leon Bichmann, Annika Nelde, Michael Ghosh, Lukas Heumos, Christopher Mohr, Alexander Peltzer, Leon Kuchenbecker, Timo Sachsenberg, Juliane S. Walz, Stefan Stevanović, Hans-Georg Rammensee & Oliver Kohlbacher
Journal of Proteome Research 2019 18 (11), 3876-3884. doi: 10.1021/acs.jproteome.9b00313
An extensive list of references for the tools used by the pipeline can be found in the CITATIONS.md file.
You can cite the nf-core publication as follows:
The nf-core framework for community-curated bioinformatics pipelines.
Philip Ewels, Alexander Peltzer, Sven Fillinger, Harshil Patel, Johannes Alneberg, Andreas Wilm, Maxime Ulysse Garcia, Paolo Di Tommaso & Sven Nahnsen.
Nat Biotechnol. 2020 Feb 13. doi: 10.1038/s41587-020-0439-x.
In addition, references of tools and data used in this pipeline are as follows:
Fred2 Immunoinformatics Toolbox
Schubert B. et al, Bioinformatics 2016 Jul 1;32(13):2044-6. doi: 10.1093/bioinformatics/btw113. Epub 2016 Feb 26
Comet Search Engine
Eng J.K. et al, J Am Soc Mass Spectrom. 2015 Nov;26(11):1865-74. doi: 10.1007/s13361-015-1179-x. Epub 2015 Jun 27.
Percolator
Käll L. et al, Nat Methods 2007 Nov;4(11):923-5. doi: 10.1038/nmeth1113. Epub 2007 Oct 21.
Identification based RT Alignment
Weisser H. et al, J Proteome Res. 2013 Apr 5;12(4):1628-44. doi: 10.1021/pr300992u. Epub 2013 Feb 22.
Targeted peptide quantification
Weisser H. et al, J Proteome Res. 2017 Aug 4;16(8):2964-2974. doi: 10.1021/acs.jproteome.7b00248. Epub 2017 Jul 19.
MHC affinity prediction
O’Donnell T.J., Cell Syst. 2018 Jul 25;7(1):129-132.e4. doi: 10.1016/j.cels.2018.05.014. Epub 2018 Jun 27.
Shao X.M., Cancer Immunol Res. 2020 Mar;8(3):396-408. doi: 10.1158/2326-6066.CIR-19-0464. Epub 2019 Dec 23.