nf-core/sarek
Analysis pipeline to detect germline or somatic variants (pre-processing, variant calling and annotation) from WGS / targeted sequencing
3.0.2). The latest
stable release is
3.7.1
.
Introduction
nf-core/sarek is a workflow designed to detect variants on whole genome or targeted sequencing data. Initially designed for Human, and Mouse, it can work on any species with a reference genome. Sarek can also handle tumour / normal pairs and could include additional relapses.
The pipeline is built using Nextflow, a workflow tool to run tasks across multiple compute infrastructures in a very portable manner. It uses Docker/Singularity containers making installation trivial and results highly reproducible. The Nextflow DSL2 implementation of this pipeline uses one container per process which makes it much easier to maintain and update software dependencies. Where possible, these processes have been submitted to and installed from nf-core/modules in order to make them available to all nf-core pipelines, and to everyone within the Nextflow community!
On release, automated continuous integration tests run the pipeline on a full-sized dataset on the AWS cloud infrastructure. This ensures that the pipeline runs on AWS, has sensible resource allocation defaults set to run on real-world datasets, and permits the persistent storage of results to benchmark between pipeline releases and other analysis sources. The results obtained from the full-sized test can be viewed on the nf-core website.
It’s listed on Elixir - Tools and Data Services Registry and Dockstore.
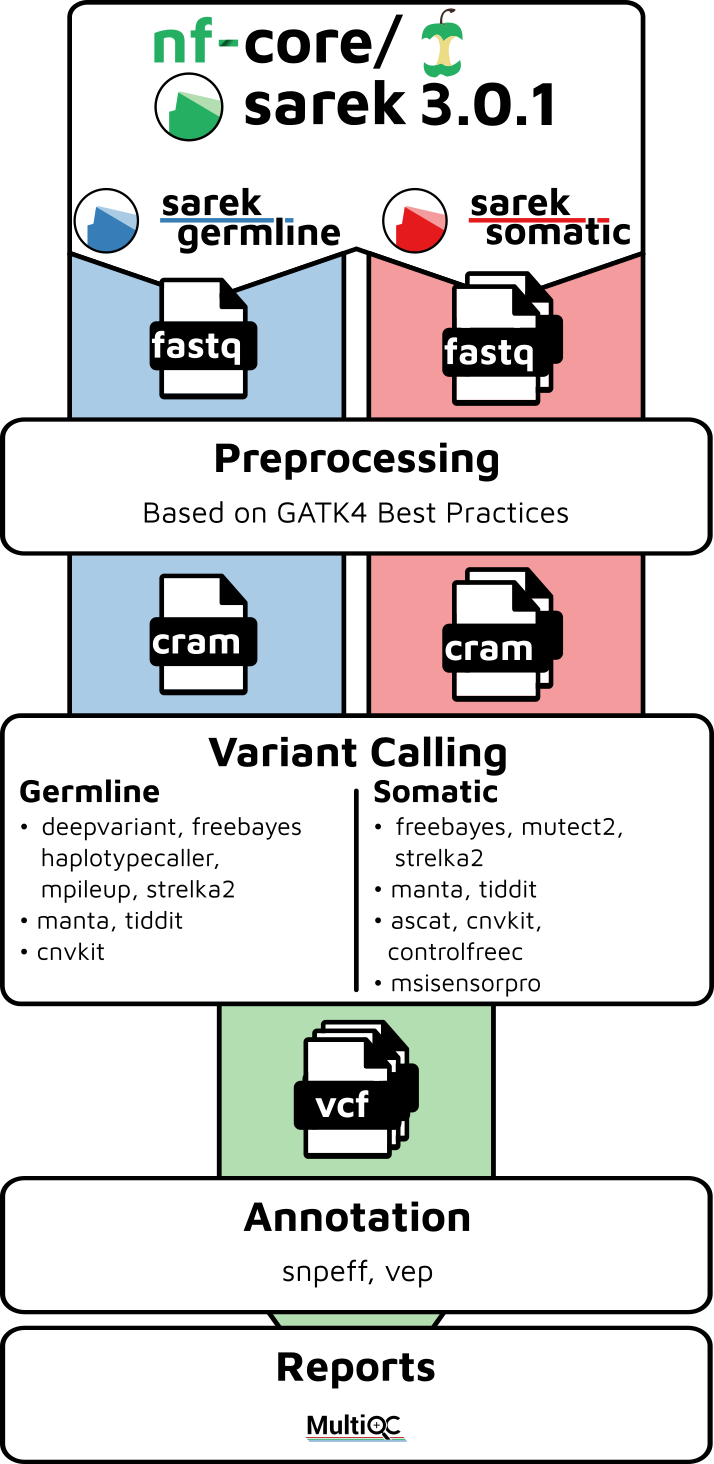
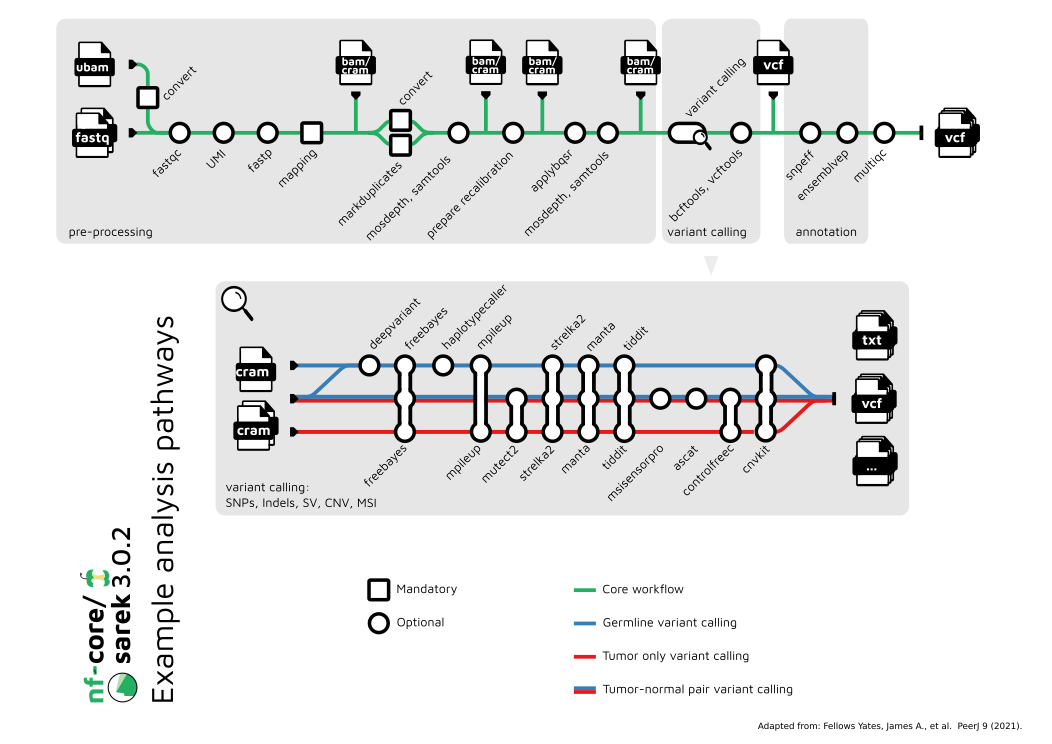
Pipeline summary
By default, the pipeline currently performs the following:
- Sequencing quality control (
FastQC) - Map Reads to Reference (
BWA mem) - Mark Duplicates (
GATK MarkDuplicates) - Base (Quality Score) Recalibration (
GATK BaseRecalibrator,GATK ApplyBQSR) - Preprocessing quality control (
samtools stats) - Preprocessing quality control (
mosdepth) - Overall pipeline run summaries (
MultiQC)
Quick Start
-
Install
Nextflow(>=21.10.3) -
Install any of
Docker,Singularity(you can follow this tutorial),Podman,ShifterorCharliecloudfor full pipeline reproducibility (you can useCondaboth to install Nextflow itself and also to manage software within pipelines. Please only use it within pipelines as a last resort; see docs). -
Download the pipeline and test it on a minimal dataset with a single command:
nextflow run nf-core/sarek -profile test,YOURPROFILE --outdir <OUTDIR>Note that some form of configuration will be needed so that Nextflow knows how to fetch the required software. This is usually done in the form of a config profile (
YOURPROFILEin the example command above). You can chain multiple config profiles in a comma-separated string.- The pipeline comes with config profiles called
docker,singularity,podman,shifter,charliecloudandcondawhich instruct the pipeline to use the named tool for software management. For example,-profile test,docker. - Please check nf-core/configs to see if a custom config file to run nf-core pipelines already exists for your Institute. If so, you can simply use
-profile <institute>in your command. This will enable eitherdockerorsingularityand set the appropriate execution settings for your local compute environment. - If you are using
singularity, please use thenf-core downloadcommand to download images first, before running the pipeline. Setting theNXF_SINGULARITY_CACHEDIRorsingularity.cacheDirNextflow options enables you to store and re-use the images from a central location for future pipeline runs. - If you are using
conda, it is highly recommended to use theNXF_CONDA_CACHEDIRorconda.cacheDirsettings to store the environments in a central location for future pipeline runs.
- The pipeline comes with config profiles called
-
Start running your own analysis!
nextflow run nf-core/sarek --input samplesheet.csv --outdir <OUTDIR> --genome GATK.GRCh38 -profile <docker/singularity/podman/shifter/charliecloud/conda/institute>
See usage docs for all of the available options when running the pipeline.
Documentation
The nf-core/sarek pipeline comes with documentation about the pipeline usage, parameters and output.
Credits
Sarek was originally written by Maxime Garcia and Szilveszter Juhos at the National Genomics Infastructure and National Bioinformatics Infastructure Sweden which are both platforms at SciLifeLab, with the support of The Swedish Childhood Tumor Biobank (Barntumörbanken). Friederike Hanssen and Gisela Gabernet at QBiC later joined and helped with further development.
Main authors:
We thank the following people for their extensive assistance in the development of this pipeline:
- Abhinav Sharma
- Adrian Lärkeryd
- Alexander Peltzer
- Anders Sune Pedersen
- Chela James
- David Mas-Ponte
- Francesco Lescai
- Gavin Mackenzie
- Gisela Gabernet
- Harshil Patel
- James A. Fellows Yates
- Jesper Eisfeldt
- Johannes Alneberg
- José Fernández Navarro
- Lasse Westergaard Folkersen
- Lucia Conde
- Malin Larsson
- Marcel Martin
- Nick Smith
- Nilesh Tawari
- Olga Botvinnik
- Oskar Wacker
- Paul Cantalupo
- Phil Ewels
- Sabrina Krakau
- Sebastian-D
- Silvia Morini
- Susanne Jodoin
- Tobias Koch
- Winni Kretzschmar
- arontommi
- BarryDigby
- bjornnystedt
- cgpu
- gulfshores
- pallolason
Acknowledgements
Contributions & Support
If you would like to contribute to this pipeline, please see the contributing guidelines.
For further information or help, don’t hesitate to get in touch on the Slack #sarek channel (you can join with this invite), or contact us: Gisela Gabernet, Maxime Garcia, Friederike Hanssen, Szilvester Juhos
Citations
If you use nf-core/sarek for your analysis, please cite the Sarek article as follows:
Garcia M, Juhos S, Larsson M et al. Sarek: A portable workflow for whole-genome sequencing analysis of germline and somatic variants [version 2; peer review: 2 approved] F1000Research 2020, 9:63 doi: 10.12688/f1000research.16665.2.
You can cite the sarek zenodo record for a specific version using the following doi: 10.5281/zenodo.3476425
An extensive list of references for the tools used by the pipeline can be found in the CITATIONS.md file.
You can cite the nf-core publication as follows:
The nf-core framework for community-curated bioinformatics pipelines.
Philip Ewels, Alexander Peltzer, Sven Fillinger, Harshil Patel, Johannes Alneberg, Andreas Wilm, Maxime Ulysse Garcia, Paolo Di Tommaso & Sven Nahnsen.
Nat Biotechnol. 2020 Feb 13. doi: 10.1038/s41587-020-0439-x.





